The Story of One Dose
Inside the sprawling operational puzzle of bringing the Johnson & Johnson COVID vaccine to the public.
Photo-Illustration: Intelligencer/Shutterstock / M-Foto
This article was featured in One Great Story, New York’s reading recommendation newsletter. Sign up here to get it nightly.
As
an object, it’s not much: an inch and a half of glass with a stopper
and some liquid inside. But a thimbleful of the stuff has amazing power —
the ability to liberate us from our yearlong collective trauma. The
fact that it’s available, scarcely a year after the start of a pandemic,
is both an industrial miracle and a freakish stroke of luck; a decade
ago, technology did not exist that could bring vaccines so quickly to
the public’s arms.
Pfizer
and Moderna crossed the finish line first, neck and neck, in December.
The third and most recently approved vaccine was from Johnson &
Johnson. The J&J vaccine holds some crucial advantages:
Only one dose is required rather than two, and while the other approved
vaccines expire 30 days after thawing, Johnson & Johnson’s lasts
three months, making it easier to distribute in countries that lack an
advanced cold chain. The story of the vaccine’s path from development to
mass distribution is a lesson in the power of the global capitalist
system — the network of corporations and supply chains that, though it
can suffocate and disempower us as individuals, can also summon forth
immense material and intellectual resources and deploy them for the
greater good.
From
the start, J&J struggled to catch a break. The pharmaceutical giant
played it safe during development and lost crucial time, failed to get
FDA approval for parts of its U.S. production chain, missed several
delivery targets, and wound up with a vaccine that underperformed its
rivals in clinical trials. Then, another obstacle: Last week, the New
York Times revealed that the new batch J&J had pledged would be delayed even further, after a mix-up at a subcontractor’s production facility ruined 15 million doses. The Biden administration has since directed J&J to take over every aspect of vaccine production at the plant.
The setback was significant, but not fatal. The facility where the mix-up occurred was
part of a production process that relies on a precise orchestration of
timing, engineering, and logistical expertise across multiple
continents, which makes it vulnerable to bad luck and human error. But
the system is also resilient: When the batch of J&J doses was
compromised, alternative supply lines were available to compensate for
the failure. Here is how that entire tempestuous journey unfolded — the
breakthroughs, the setbacks, and the way the pieces came together to
bring vaccines to millions of arms.
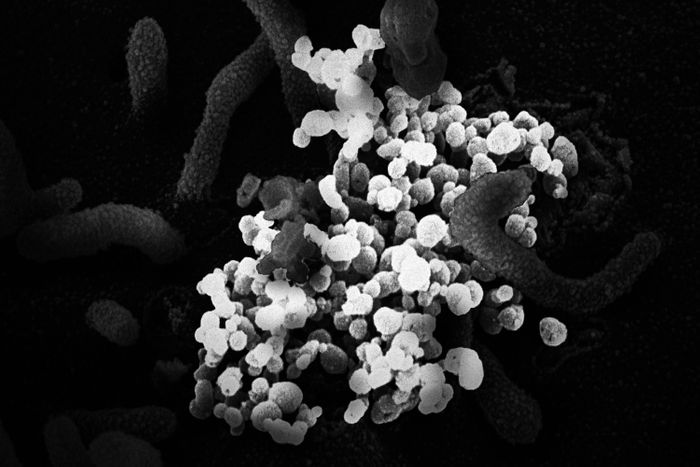
Photo: IMAGE POINT FR/NIH/NIAID/BSIP/Un
On
the afternoon of January 3, 2020, a box arrived at the laboratory of
the Shanghai Public Health Clinical Center, a complex 30 miles southwest
of the city center. The box contained swabs taken from a patient in
Wuhan who had fallen ill with a new kind of pneumonia. Worried about how
dangerous the new pathogen might turn out to be, the Chinese government
had banned medical researchers from publishing any information about
it. China had experienced public-health scares before: Between 2002 and
2004, a deadly and highly infectious virus called SARS had spread
through the country. It was in response to SARS that the Shanghai Public
Health Clinical Center had been built.
Zhang
Yongzhen, the laboratory’s leader, was a specialist in assessing new
viruses. Over the next 40 hours, his team painstakingly broke the
genetic code of the new pathogen into its sequence of nucleotide base
pairs — essentially decoding the software that the virus plugged into
its host to make copies of itself. The sequence would tell researchers
exactly how it worked so that they could figure out how to thwart it.
At 2 a.m. on January 5, according to a profile in Nature,
one of Zhang’s team members gave him bad news: The virus was a close
relative of SARS. Though little was yet known about how the disease
progressed or how it was transmitted, the potential for rapid spread and
widespread death was real.
On
the morning of Saturday, January 11, Zhang was on a plane about to take
off for Beijing when he got a call from Edward Holmes, a virologist
from the University of Sydney. Holmes, a longtime colleague of Zhang’s,
knew that he had sequenced the virus’s genome. Holmes impressed upon his
colleague the importance of publishing the information. Zhang asked for
time to think. If the new disease was as contagious as SARS, it could
spread beyond China and put the whole world at risk. But there was a
more immediate hazard: the danger of angering Chinese authorities. An
airline attendant appeared and told Zhang that the flight was about to
take off. He had to decide. “Okay,” Zhang said. Holmes could release the
sequence data.
In
Boston, on the other side of the international dateline, it was still
Friday, January 10. Dan Barouch, a virologist at the Beth Israel
Deaconess Medical Center, was hosting an off-site meeting with his lab
at the Boston Museum of Science, in a room that enjoyed a view of the
Charles River. Barouch and his team had gathered to plan for the year
ahead, but everyone was discussing the news from China, where the first
death linked to a new form of pneumonia had just been announced. “It had
all the hallmarks of a virus that we thought might have pandemic
potential,” Barouch recalls.
In
some ways, this was the moment Barouch had been preparing for. For the
last decade and a half, he and his team had been developing a “vector” —
a way to sneak part of a pathogen’s genetic code into human cells. Once
there, it would trigger the cells to create pieces of the pathogen for
the body’s immune system to identify. Their vector was a variant of an
adenovirus, a bug that causes the common cold. Called Ad26, it had had
several of its genes removed so that, while it was able to insert itself
into human cells, it couldn’t reproduce and make a person sick. But it
could still merge with the human host cell and bring with it the
pathogen DNA needed to cue the immune system. This approach could
theoretically work with almost any infectious agent — Barouch’s team had
already used Ad26 to make vaccines for HIV, tuberculosis, and Zika.
Pfizer and Moderna would follow a similar technique using mRNA; by using
DNA, a more stable nucleic acid, Barouch & Co. were crafting a
vaccine that could be injected in one dose.
It
was Friday evening, Boston time, when Zhang’s data hit the internet.
Zhang’s lab had decoded the virus into letters symbolizing the four base
pairs that make up its genetic code: A, C, G, and T. In effect, Zhang
and his team had compressed a living thing into pure information. By
uploading it, they had transformed it again, into a string of ones and
zeros split up into packets that bounced around the fiber-optic nodes of
the internet. Barouch and his team went to work. On Monday, they began
translating Zhang’s digital data back into nucleotide sequences, which,
in turn, could be converted back into an actual virus. It was as though
pieces of the coronavirus had teleported through the web.
Immediately,
Barouch saw the best strategy. He noticed that the virus contained a
spike protein — a piece that sticks out like the rubber hair on a Koosh
ball, which an antibody in the human bloodstream would be most likely to
recognize. From the genome, Barouch could tell which stretch of DNA
coded for it. To make the vaccine, this would be the sequence to pack
into the Ad26 vector. The question was what exactly the message should
contain: the whole spike protein sequence or just part? Should the
scientists include a preamble, or other sections that might help get the
message across to the immune system? The decision could mean the
difference between an efficacious vaccine and a useless one.
Meanwhile,
the disease was spreading rapidly in China and cases were starting to
appear internationally. Barouch contacted Johan Van Hoof, the head of
vaccine development at Janssen Pharmaceuticals, a subsidiary of Johnson
& Johnson with R&D centers in Leiden, the Netherlands. They had
worked together on vaccine development for more than a decade. “Johann,
this is looking bad,” Barouch told him. “I think we need to make a
vaccine.”
“Absolutely,” Van Hoof said. “We do.”
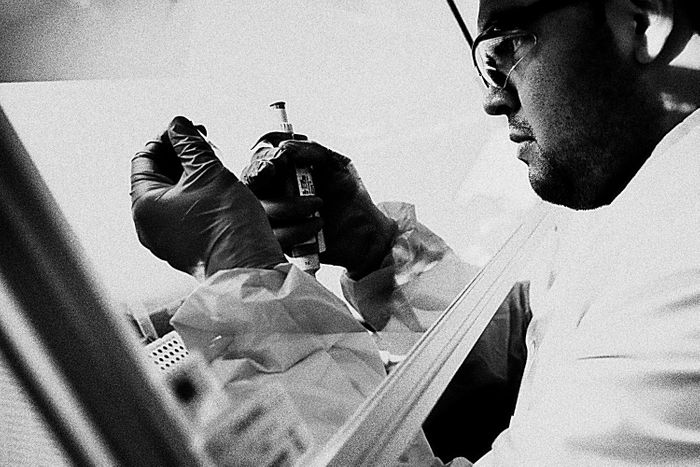
Photo: Craig F. Walker/The Boston Globe
The
labs in Boston and Leiden began working in parallel, staying in touch
with daily phone calls. They started by creating a dozen different
lengths of DNA and injecting them directly into mice to see which
triggered the most vigorous immune response. They then winnowed the list
to seven candidates, packed them into the Ad26 vector, and tested the
variants on Rhesus monkeys. It was a bit like A/B testing of different
versions of an Internet ad: Each version of the DNA snippet would cause
the body’s cells to produce a different protein, which, in turn, would
have a different effect on the immune system. They wanted to make sure
they chose the most powerful one.
On
February 11, the worldwide death toll passed 1,100, with more than
44,000 cases reported in China and several other countries. Health
officials at last gave the disease a name: COVID-19. In those crucial
early months, every day in the lab presented an existential battle
between quality and speed. If Barouch and Van Hoof made a best guess at
the right sequence and put it straight into the vector, they could roll
out the vaccine sooner and save countless lives — the approach that
Moderna and AstraZeneca chose. But this could result in an inferior
vaccine that would allow more people to die. In the end, the scientists
decided to take a few months to test multiple versions on animals first,
then advance the most effective one to human testing.
They
ran the test on 52 animals. Some of the variants were given to four
animals, others to six. The researchers then exposed all the test
animals to COVID. Compared to control animals, the monkeys that received
versions of the vaccine showed little viral replication. But monkeys
that received a variant called S.PP showed almost no sign of infection
at all. On March 30, 2020, it was this variant that Janssen announced as
its vaccine candidate. It was called Ad26.COV2.S.
To
ensure that the Ad26.COV2.S would be safe and effective for humans, the
company would need to test it — first on a few hundred people, then on
tens of thousands. It normally takes years to conduct a full slate of
tests, get results, and design a follow-up round. Instead, Van Hoof’s
team began running steps in parallel. As soon as it could see what
direction the animal tests were going, the lab started to produce
vaccine material for human trials — a decision that would move the trial
start date up from September to July. And, in April, even before the
results of the clinical trial came in, Johnson & Johnson began
making plans to manufacture and package the vaccine at scale, so that
mass quantities would be ready by the time the human tests came back. If
the results were good, J&J could quickly start putting needles in
arms. If not, the vaccines would get chucked in the trash, and billions
of dollars spent on research and development would go up in smoke.
Months later, Johnson & Johnson learned it had made the right call. Clinical-trial results showed the vaccine worked and was safe, and it received a green light from the FDA in February 2021.
Photo: Michael Robinson Chavez/The Washington Post
Johnson
& Johnson makes a wide variety of medications, but it had never
fulfilled an order as large as the coronavirus vaccine, a drug that 7.8
billion people — 331 million in the U.S. alone — needed right away. By
spring, the Trump administration was lobbing contracts at
vaccine-makers: $483 million to Moderna, $456 million to Johnson &
Johnson, $30 million to Sanofi, then $1.2 billion to AstraZeneca, and
$1.95 billion to Pfizer. Johnson & Johnson promised 100 million
doses, but even that was way beyond its capabilities. So to augment
production from Janssen’s own facility in Leiden, it signed up partners
around the world to produce its as-yet-unproven vaccine, including, in
the U.S., a company called Emergent BioSolutions.
Founded
in 1998 to produce anthrax vaccine as a defense against terror attacks,
Emergent had, over the years, received hundreds of millions of dollars
to provide doses for the U.S. government’s strategic stockpile. When the
threat came in the form of a pandemic, it was tasked, instead, with
manufacturing millions of doses of Ad26.COV2.S. But growing Ad26 viral
vectors wasn’t a straightforward process. To turn the original virus
into a harmless vaccine, researchers had deleted genes the virus needs
for replication; in order to make copies of itself, the Ad26.COV2.S
vector required a special environment. The solution was to insert the
virus’s missing genes into a unique human-cell line that had originated
in the eye of a human fetus aborted in the mid-1980s. This genetically
modified cell line was named PER.C6. Unlike normal human cells, which
can multiply only so many times before dying, these cells are immortal;
as long as they’re fed the right nutrients and kept at the right
temperature, they can grow forever. Because PER.C6 contained the genes
that Ad26 needed to reproduce, it held the key to growing the viral
vectors.
Last
fall, in an office park in East Baltimore, Emergent technicians added
the contents of a cell-culture bag to a ceiling-high tank holding a
single-use bioreactor. The bioreactor was filled with a 1,000-liter
solution of sugars, proteins, and other nutrients. Inside the warm bath,
cells began to multiply.
Once
the PER.C6 cells grew to the right volume, workers added several liters
of Ad26.COV2.S seed containing millions of vector particles. Inside the
bioreactor was a genetically engineered paradise. The adenovirus
particles latched onto the exterior of PER.C6 cells and injected them
with their DNA. Once inside, the genetic material caused the cells to
start manufacturing the components that make up the virus particle,
until the hosts were so stuffed with tiny self-assembled machines that
they burst, spewing out a conquering robot army into the broth. After a
week, the PER.C6 cells were either hijacked or dead,
and the tank contained quadrillions of virus particles, enough for
millions of doses. Waste and cell fragments got filtered out, and what
was left was concentrated, then frozen at 94 degrees below zero. The
resulting material is known as vaccine substance.
For
months, this process took place again and again in Baltimore and in the
Netherlands. But by early 2021, the millions of units of vaccine
substance produced at Emergent still weren’t allowed to leave the plant.
Like Moderna and Pfizer, J&J, under the pressures of a global
crisis, had begun activating parts of its supply chain before all of
them had received the official green light, and the FDA had not yet
inspected and approved the Baltimore facility, which had, in recent
years, received a string of citations for quality-control issues.
Then,
in late February, disaster struck. Emergent accidentally mixed Johnson
& Johnson vaccine ingredients with those of AstraZeneca’s,
destroying 15 million doses and further delaying the facility’s FDA
approval and the delivery of more vaccines. The plant had caught the
error and quarantined the incorrect doses, and, in the meantime, a new
supply line was starting to get under way — in March, President Biden
announced that the pharmaceutical giant Merck, which had abandoned its
own vaccine initiative, would convert and upgrade its facilities to help
manufacture more J&J doses. But until it could sort out the mess,
Johnson & Johnson was forced to rely on vaccine substance produced
at Janssen’s plant in Leiden.
Photo: Catalent Pharma Solutions/Youtube
After
the vaccine substance is manufactured and approved, it then has to go
to a “finish and fill” facility, where the deep-frozen concentrate will
get turned into stuff that can actually be injected into a person. Last
year, J&J scrambled to contract with Catalent Biologics, an
international pharmaceutical processing company that could quickly
expand its plant in Bloomington, Indiana. In June, Catalent was also
tapped to finish-and-fill the Moderna vaccine.
Inside
a space that, a year ago, was an empty warehouse, gleaming panoplies of
stainless-steel arms and wheels whir. Behind aseptic barriers, the
vaccine substance is thawed, blended with substances like
2-Hydroxypropyl-β-cyclodextrin (to improve its solubility) and
Polysorbate 80 (a stabilizer often used in food and cosmetics), then fed
into a vial-filling assembly line. Sterilized vials move by conveyor
belt into a filling station where a multipronged needle bobs up and
down, squirting ten full at a time.
There
is no margin for error. A single vial that gets chipped or cracked
could ruin an entire production run. Optimizing this kind of process is a
never-ending task, with chemists and engineers tuning and tweaking at
the nano level. The material sciences company Corning, for instance,
recently figured out how to prevent glass from flaking off vials by
switching from borosilicate to aluminosilicate, a substance it calls
Valor Glass.
Each
newly filled vial contains five doses — about a quarter-trillion
adenovirus particles in three milliliters of liquid. A printer lays an
alphanumeric code around the vial, then a robotic packing machine places
ten vials in a box, at a rate of 600 vials a minute. During the filling
process, no human hand has intervened once. As with Emergent,
Catalent’s new facility needed an FDA signoff before the material it
produces could be released to the public — on March 23, 2021, that
approval finally arrived. Five days later, the Bloomington plant began
shipping out J&J vials, which could then join the 6 million finished
vaccines that had been sent to the U.S. from J&J’s Netherlands
factory.
Late
last year, a billboard appeared along the highway in Shepherdsville,
Kentucky, a rural town 20 miles south of Louisville. “NOW HIRING,” it
said. “Material Handlers Get Paid Up to $20.12 Per Hour.” The jobs were
posted by McKesson, a large pharmaceutical-distribution company that had
been contracted by the Centers for Disease Control and Prevention to
package and dispatch all COVID-19 vaccines, except for Pfizer’s. In late
2020, the company completed construction on its brand-new
Shepherdsville facility, a squat, quarter-mile-long million-square-foot
warehouse, and it needed to hire more than 500 workers.
In
the race to create and distribute the vaccine, McKesson’s role may
first appear as mundane as that of any fulfillment center: It’s tasked
with counting out the correct number of ten vial-size boxes for each
site (900 vaccine doses to a high school in Kalamazoo, Michigan, say, or
300 to a health center in Athens, Georgia) and packaging them into
cartons for shipment. But it’s the scale of the operation that’s key — a
huge portion of America’s vaccine production will, at one point, need
to go through McKesson. Its Shepherdsville hub is one of the company’s
four facilities shipping COVID vaccine doses; to construct the storage
racks alone required at least 3.7 million pounds of steel, more than a
quarter of the metal frame used to construct the Eiffel Tower.
Inside
a hangar-like room hung with American flags, workers load the boxes of
J&J vaccines into KoolTemp EPS coolers, adding frozen gel packs and a
monitoring device that logs the temperature and triggers a warning if
it gets too high or low. To prevent that from happening, workers are
allotted 30 minutes to place the coolers into cartons. (In another
section of the facility, Moderna doses, which must be maintained at a
lower temperature, are packed inside a freezer by workers wearing parkas
and insulated boots.) McKesson workers are also tasked with assembling
the kits that contain everything a vaccination center needs to
administer the vaccines: alcohol pads, face shields, surgical masks, and
needles and syringes.
Less than two days after arriving at the McKesson warehouse, the vaccine is off on the next leg of its journey.
A
UPS semi pulls up to the loading dock, and workers place the KoolTemp
cartons into the trailer. The truck swings onto the onramp to I-65 North
and drives the 20 miles to UPS Worldport, a 5.2-million-square-foot
intermodal shipping hub located on the grounds of Louisville Muhammad
Ali International Airport. There, the boxes are unloaded, scanned, and
sorted through a 155-mile-long system of conveyor belts.
Until
now, each stage of the vaccine-making process has required some kind of
scientific or logistical coup: the compression of a years-long process
into a matter of weeks, the creation of a new technology, the rapid
alteration of a physical landscape. But when it came time to move those
millions of doses to their final destinations, it took barely any extra
effort for UPS and FedEx to activate their networks. After all, among
the 20 million packages delivered daily, thousands of vaccine shipments
were a rounding error.
In
2019, UPS introduced a service for health-care customers that allows
them to more precisely track shipments of medicines, samples, and
vaccines. Each carton is affixed with a tag that allows sensors to track
its location, and the data gets transmitted to the UPS health-care
command center inside Worldport, a room with four large-screen monitors
on the wall displaying national weather and UPS flights in transit. When
bad weather before Christmas meant that a vaccine shipment’s
destination was closed, for instance, the health-care command center got
on the phone and arranged to have it redirected.
Photo: Timothy D. Easley/POOL/AFP
As
the cartons wend their way through the maze of conveyor belts, scanners
read the label on each and shunt it to the appropriate outbound loading
station. Shortly before midnight, planes start arriving at the
Louisville airport at the rate of about one a minute, carrying inbound
packages; those packages get sorted at the rate of 115 every second,
then loaded back onto planes heading for their final destination.
Within
15 minutes of its unloading at Worldport, a box slides down a chute to
the outbound station. A worker scans it and checks all six sides for
damage, then loads it into an airfreight shipping container bound for
New York. Around 4 a.m., the pilot takes off.
Photo: Johnny Milano/Bloomberg via Getty Images
Later
that morning, the box arrives at the loading dock of a vaccination
site. This final leg of the vaccine’s journey — delivering doses to a
place where they can be put into people’s arms — is the simplest in
concept, but was, for a long time, the most vexing. In the weeks after
the first vaccines were approved and shipped, New York State proved
ill-prepared to deliver its allocated doses. By early January, some
vaccination sites had used less than 20 percent of the doses they’d been
sent.
Similar
problems played out across the country. Despite having lavished
billions on producing vaccines, the Trump administration had largely
ignored the question of distribution and administration, leaving the
matter to states and offering little support. Entire organizational
structures had to be built on the fly using whatever labor happened to
be available. In New York City, the effort leaned heavily on the Medical
Reserve Corps, a group of more than 15,000 volunteers who stand by to
help out in crises. By the end of January, the system was running
smoothly enough that the vaccination centers were efficiently doling out
what they’d received.
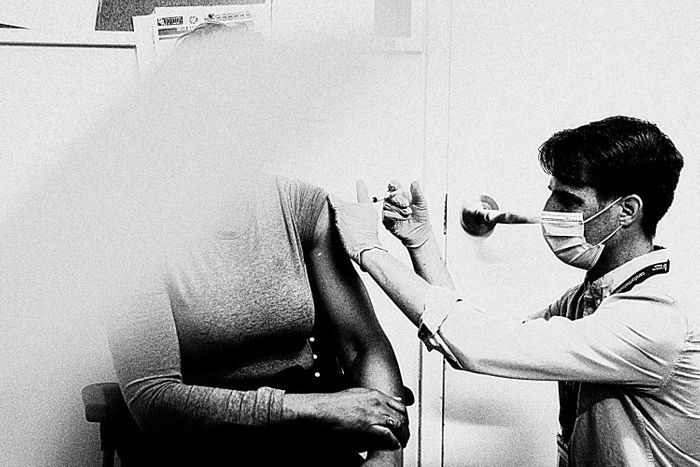
Photo: Angus Mordant/Bloomberg via Getty Images
When
it’s your turn, you roll up your sleeve. The nurse chooses a needle
based on the heft of your arm — an inch and a half for larger people, an
inch otherwise — and pushes the tip into the rubber gasket atop the
vial containing your dose. She slips the needle into your muscle. Inside
your arm, 50 billion genetically engineered nanoscale robot assassins,
carrying genetic payloads downloaded from the internet and bred in a
soup of immortal human-eye cells, begin prying their way through your
cells’ defenses.
One Great Story: A Nightly Newsletter for the Best of New York