FDA Approves Anti-Malarial Drugs Chloroquine And Hydroxychloroquine For Emergency Coronavirus Treatment
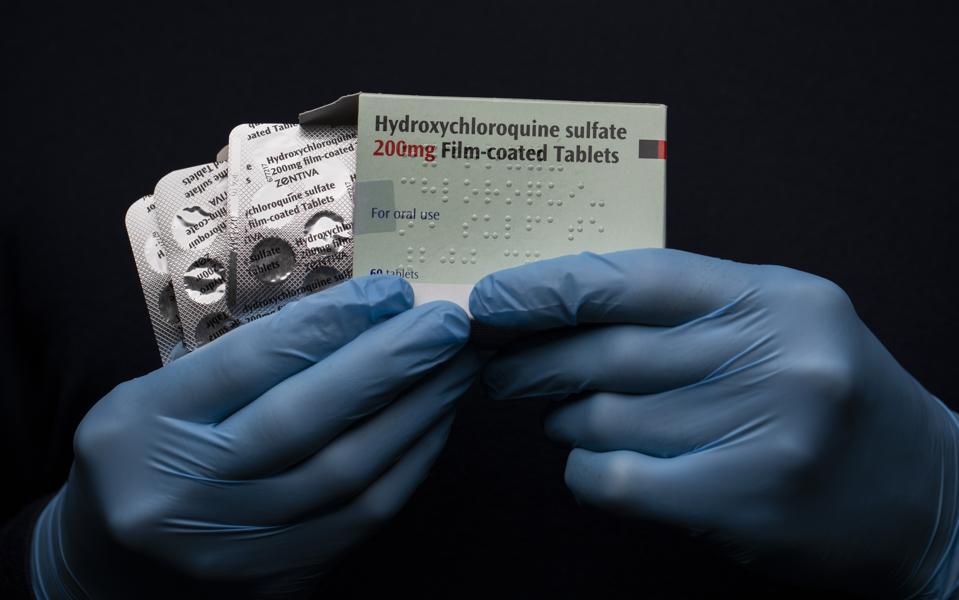
A blister pack of Hydroxychloroquine sulfate pills is displayed on March 26, 2020, in London.
John Phillips/Getty Images
Topline: The Food and Drug Administration on Sunday issued an emergency authorization for experimental coronavirus treatments using chloroquine and hydroxychloroquine, anti-malaria drugs touted by President Donald Trump despite inconclusive clinical proof of their efficacy.
- The Department of Health and Human Services said Sunday hydroxychloroquine and chloroquine products can be distributed and prescribed by doctors through the Strategic National Stockpile “to hospitalized teen and adult patients with COVID-19, as appropriate, when a clinical trial is not available or feasible.”
- HHS said Germany’s Sandoz has already given 30 million doses of hydroxychloroquine to the Strategic National Stockpile, the federal government’s inventory of medical supplies for public health emergencies, while Bayer has donated a million doses of chloroquine.
- The agency is fast-tracking a process that usually takes years while the FDA conducts clinical trials in New York, a hot spot for the virus.
- HHS said the emergency authorization was issued because the potential benefits of the product outweigh the risks and acknowledged that “anecdotal reports suggest that these drugs may offer some benefit in the treatment of hospitalized COVID-19 patients,” but cautioned that “clinical trials are needed to provide scientific evidence that these treatments are effective.”
- Trump had falsely claimed that the FDA had approved the drugs for coronavirus treatment before Sunday.
- Confusion over their use has led some Americans to seek over-the-counter replacements, such as an Arizona man who bought a nonpharmaceutical form of chloroquine phosphate, a common chemical used to clean fish tanks, which killed him and landed his now-widow in intensive care.
- The CDC warns against taking nonpharmaceutical chloroquine phosphate without a prescription and the supervision of a healthcare provider because it “can cause serious health consequences, including death.”
“It’s a difficult issue because it’s an emotional issue. With people having no other options available, it’s hard to say that if there’s something that seems like it may work, they should be denied the option to try it. The part that makes this more complicated is that there was an irresponsible, in my opinion, expression that this actually is effective when there is no real scientific evidence that it is,” Dr. Kenneth Kaitin, director of the Tufts Center for the Study of Drug Development, told Forbes.
Today In: Forbes
News peg: Scientists hope chloroquine and hydroxychloroquine—decades-old drugs that are used to treat malaria, lupus and rheumatoid arthritis—may be used to treat the coronavirus, but early studies have provided mixed evidence proving their effectiveness and the drugs may entail risks such as vision problems or cardiac arrest. The frenzy surrounding the treatment has caused some doctors to hoard hydroxychloroquine—which is sold under the brand name Plaquenil—by writing prescriptions for themselves or for their families. Some state pharmacy boards have issued rules limiting prescriptions, including Texas, Louisiana, Ohio and North Carolina.

No comments:
Post a Comment